PRODUCT
Specifications
There are no special storage conditions required. Please refer to the radiation safety regulations of your country.
ACTIVITY
2.5 ml containing 185 MBq
5.0 ml containing 370 MBq
HALF LIFE I-123
13.2 hours
SHELF LIFE
2.5 ml // 7 hours from the reference time stated on the label
5.0 ml // 20 hours from the reference time stated on the label
Reference time: 01:00 p.m. CET on the day of delivery
GLOBAL
Logistics and Sales
High flexibility. 2.5 ml and 5.0 ml are manufactured from the same batch.
DAYS OF DELIVERY
Upon request
ORDER DEADLINE
3 working days before delivery
CALIBRATION TIME
01:00 p.m. CET on the day of delivery
STORAGE
This medicinal product does not require any special conditions. Local radiation protection regulations must be followed.
ABOUT ROTOP
Our Manufacturing Site
ROTOP Radiopharmacy is located in the former PET center of the Helmholtz-Zentrum Dresden-Rossendorf, Germany, and was inaugurated in 2020. In the modernized building, there are three hot cells in which high activities of Iodine-123 can be processed and Ioflupane (123I) is manufactured under cGMP conditions. The proprietary API was developed and produced entirely in-house. This precursor is used for Ioflupane production and provided to third parties.
Ioflupane (123I) ROTOP is distributed in environmentally friendly Type-A packaging all over Europe and we hold marketing authorizations in more than ten European countries. In compliance with high radiation protection standards, we developed fully automated cGMP labeling and packaging software to package and deliver Ioflupane from Dresden, Germany.
CONTACT
Let’s Talk.
If you want to order Ioflupane please contact: ioflupane@rotop-pharmaka.com or contact our customer service team: customer.service@rotop-pharmaka.com.
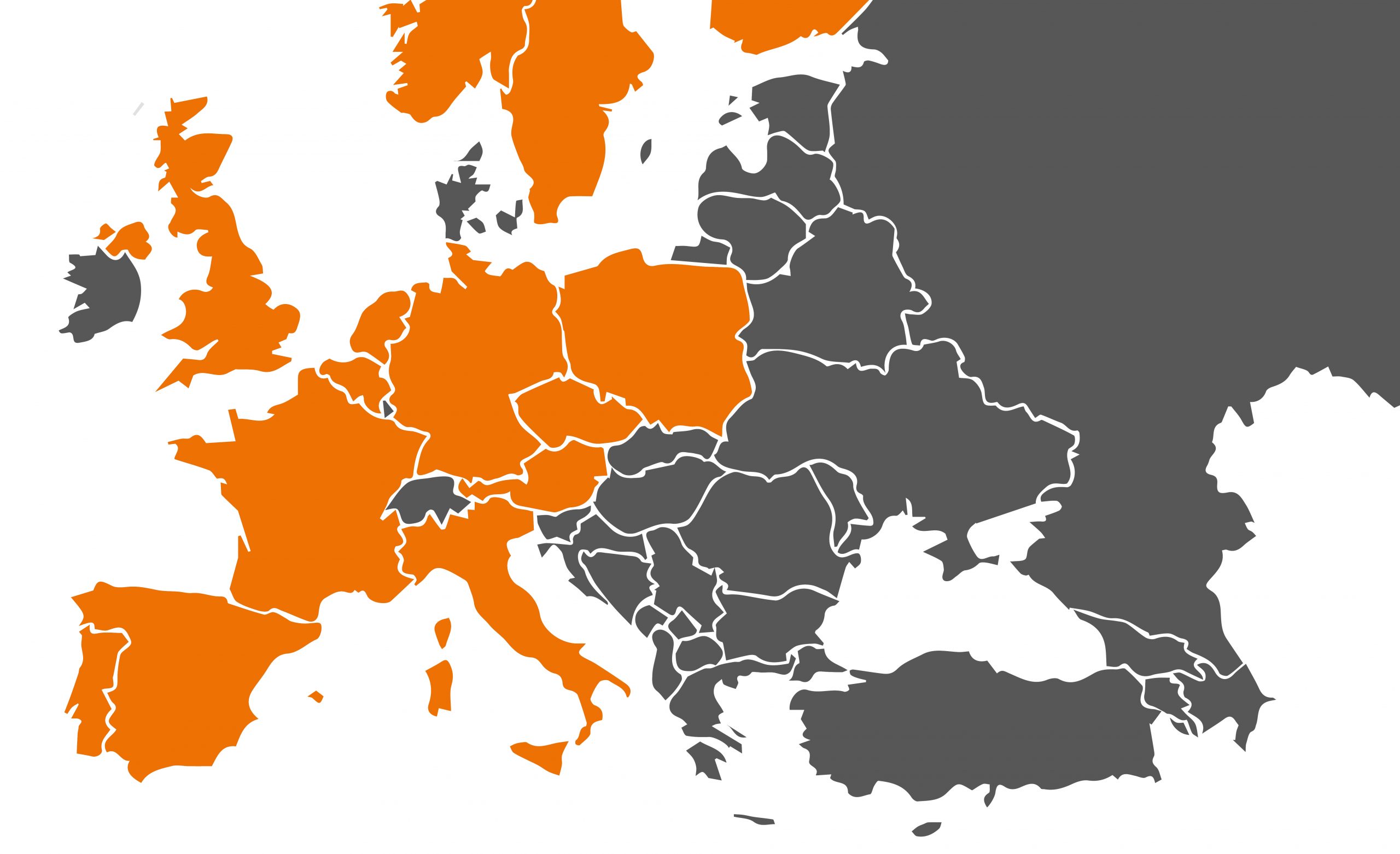
Ioflupane (123I) ROTOP is registered in the following countries:
Pharmacovigilance
By reporting adverse drug reactions (ADRs), including suspected cases, you can help provide more information on the safety of this medicine. Please contact our Pharmacovigilance department: pharmacovigilance@rotop-pharmaka.com.
CONTACT
Let’s talk.
If you want to order Ioflupane please contact: ioflupane@rotop-pharmaka.com or contact our customer service team customer.service@rotop-pharmaka.com
Ioflupane (123I) ROTOP is registered in the following countries:

Pharmacovigilance
By reporting adverse drug reactions (ADRs), including suspected cases, you can help provide more information on the safety of this medicine. Please contact our Pharmacovigilance Department: pharmacovigilance@rotop-pharmaka.com